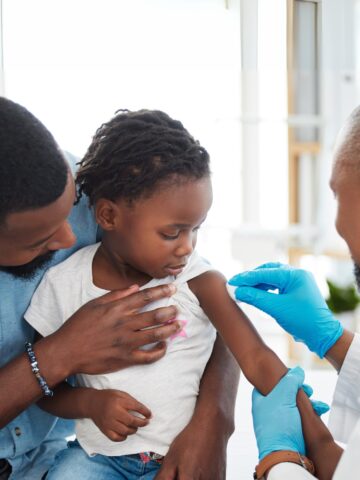
Clinical trials involving real patients help doctors and researchers learn the effectiveness of a new drug or medical device, and lead to advancements with potential to improve thousands of lives.
At any time, CHOC Children’s staff doctors, nurses and others are involved in about 300 clinical trials in many specialties, says Dr. Antonio Arrieta, director of infectious diseases and the director of infectious disease clinical research at CHOC.
These trials examine the safety and effectiveness of new medications, vaccines and medical devices as CHOC research physicians and their staffs seek to answer medical questions and develop new or improved drugs that can help children worldwide.
“CHOC is one of the best hospitals in the country and in the world when it comes to providing clinical care,” says Dr. Arrieta. “We also are seeking to make CHOC equally a leader in the discovery and development of new medications and medical devices for children.”
Dr. Arrieta and his team in are assessing the impact of a new and improved vaccine that would reduce invasive Pneumococcal disease, which can cause serious illnesses like pneumonia, meningitis and others.
Dr. Arrieta recently presented data from this research to the international community at a conference in Dublin hosted by the European Society for Pediatric Infectious Diseases and attended by more than 3,000 clinicians, researchers, residents and students.
“We want to share this with the international community,” Dr. Arrieta said. “This infection is of greater severity and more common in third-world countries that don’t have this vaccine.”
Orange County has seen a decline in cases of children with invasive Pneumococcal disease since the first vaccine was released in 2010. It is given to babies at the ages of 2, 4, 6 and 18 months.
Another area of research Dr. Arrieta’s team is working on involves an effort to treat a severe form of fungal infection in infants called candida, which can be fatal for premature babies.
“The current standard of care of is quite toxic so we are involved in an international trial involving a new agent that we think is going to improve the overall outcome of these babies,” he says.