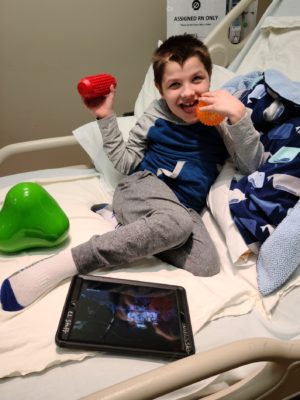
Rocio Macias was driving when her 4-year-old daughter, Isabella, coughed from the backseat.
“Are you OK?” Rocio asked.
“I’m OK,” Isabella answered with a giggle.
While likely mundane for many parents, this exchange is the stuff dreams are made of for Rocio, the mother of a child with a devastating rare disease.
“She’s doing well – she’s trying to talk a lot more,” Rocio says. “I see things physically too – she doesn’t fall as much as she used to. She doesn’t bang her head much anymore.”
Isabella is in the early stages of a clinical trial at CHOC that will evaluate a drug intended to treat a rare pediatric disease called MPS IIIA. The condition is a type of Mucopolysaccharidosis, or MPS, a genetic condition that causes physical abnormalities in young children and causes them to lose their neurological development.
Also called Sanfilippo syndrome, its early symptoms can mirror those of autism. Unlike autism, though, the patients don’t improve. Instead, they gradually deteriorate until memories and even basic abilities are lost. Most Sanfilippo patients don’t survive to adulthood.
“You ask, ‘What is that? What can I do? What can be done?’ For this one, there’s no cure. And you just cry,” Rocio says.
But Rocio found a glimmer of hope in the trial, Dr. Cristel Chapel-Crespo, Isabella’s CHOC metabolic specialist at CHOC, and Dr. Raymond Wang, also a CHOC metabolic specialist who is the director of CHOC’s Foundation of Caring Lysosomal Storage Disorder Program.
Joining the clinical trial
Phases II and III of the trial are being conducted by Lysogene, the French company that developed the experimental treatment. CHOC is one of four U.S. hospitals taking part, with several other sites in Europe.
Those patients are hard to find. In his decade of researching MPS and seeing patients, Dr. Wang estimates he’s only diagnosed 10 cases. However, given the deep heartbreak that Sanfilippo syndrome can cause with parents of affected children, Dr. Wang sought out Lysogene as a partner to provide families with access to clinical trials – and hope.
Lysogene, in turn, sought out Dr. Wang for the trial because of his expertise in researching and diagnosing the various MPS types. If the Lysogene drug is eventually approved by the U.S. Food & Drug Administration, CHOC should become the first facility on the West Coast to be able to both diagnose the disease and administer the drug, which is surgically inserted into brain tissue.
For Isabella, participating in the study meant traveling to New York, where she received the medication in June 2019.
Following a seven-hour surgery, Isabella spent a night in the hospital’s intensive care unit. The family stayed in New York for another week of tests, and then returned home.
After one more check-up in New York, the family began follow-up treatment in December much closer to home at CHOC with Drs. Chapel-Crespo and Wang, and will continue to do so every three months for five years.
“She has no after-effects at all from the surgery, is happy and doing well,” Dr. Wang says, adding that Isabella will continue to take anti-rejection medication throughout the first year of the study.
“We are hoping to prevent regression at the least, and ideally see developmental progression,” he says.
The effects of Sanfilippo
MPS IIIA, or Sanfilippo, is a subtype of MPS that affects about one in every 100,000 children. Overall, seven different types of MPS have identified: I, II, III, IV, VI, VII and IX, not counting the subtypes within them.
MPS is an inherited disease. All the types are collectively known as “lysosomal storage diseases.” Lysosomes are compartments in cells that break down molecules and remove waste products.
Normally, different enzymes in the lysosomes break down complex sugars called glycosaminoglycans, also known as mucopolysaccharides. In MPS, glycosaminoglycans are not broken down because of a deficiency in one of those lysosomal enzymes. As a result, the glycosaminoglycans accumulate in the cells and cause tissue damage.
Physical symptoms can include thickening of the lips and skin, enlarged liver and spleen, hernias, recurring ear infections, joint pain and stiffness, and shortness of stature. With Sanfilippo, which attacks brain cells, cognitive impairment could include delayed speech. Since by itself a speech delay isn’t uncommon in children, Sanfilippo’s initial symptoms only add to the confusion for families.
In the first two to three years of a patient’s life, “there might not be any symptoms,” Dr. Wang says. “Nobody ever thinks ‘My kid has Sanfilippo,’ and few doctors think about it. But it starts to be around age 3, 4, 5, when hyperactivity starts, and there are questions of autism, and usually what happens is a physician recognizes that kids with Sanfilippo look a little different.”
A mother’s instinct
Rocio began noticing some symptoms in Isabella when the toddler was about 2. Rocio thought she could explain Isabella’s unsteady walk and slow speech, but that her daughter wouldn’t outstretch her arms to catch herself when she stumbled was especially alarming.
“As a parent, you start looking around at the other kids in the class and think, ‘Is there where the other kids are too?’” Rocio said. “I never looked at it like something was wrong – I just thought she was delayed.”
As Rocio pursued speech and physical therapy for Isabella, CHOC otolaryngologist Dr. Kevin Huoh was separately evaluating Isabella for her snoring. After observing some of Isabella’s physical features, Dr. Huoh quickly referred the family to CHOC’s genetics team. Subsequent genetic testing revealed her condition.
Learning the diagnosis was devastating for Rocio. After taking the call in an empty office at work and bursting into tears, she needed to leave early for the day, she recalls. With her husband in an all-day training, Rocio was forced to deliver the news by text message.
The medication’s hope
The news was made especially frightening when the couple learned there was no treatment for Sanfilippo.
While enzyme-replacement therapy has successfully treated some types of MPS, it only works if the disease is not located in the brain. Unfortunately, the life-threatening symptoms of Sanfilippo are caused by effects of the disease in the nervous system.
Inside the brains of children with Sanfilippo syndrome, a waste product called heparan sulfate builds up, causing nerve damage and, over time, the death of nerve cells.
The Lysogene drug includes a package called a “vector.” It contains genetic instructions that enable treated nerve cells to make the missing enzyme, called sulfo-hydrolase, which clears out the waste product.
“Short-term, you can measure things like, is the body producing sulfo-hydrolase enzyme; is there a reduction in heparan sulfate?” Dr. Wang says. “But the more relevant question is, is the investigational treatment actually helping these children? What parents really care about is, Is it helping my child’s neurologic function? Is my child not regressing? Is my child progressing normally? If there were lost developmental milestones, is my child maybe even gaining them back?”
Moving forward
While Rocio has already anecdotally noticed some improvements in Isabella, time will tell whether the drug is truly effective. In the meantime, the family has found additional support through the rare disease community. They attended a local event for families impacted by MPS. Isabella was the only child with MPS IIIA.
They’ve also found some solace online, though Rocio participates only in measured doses.
“It’s hard. Sometimes I tell my husband I don’t want to follow the groups anymore,” she says. “People post when someone passes away – and it seems like that happens every day. The other day it was an 8-year-old, and you think, ’Shoot, Isabella is 4,’ and that’s heartbreaking. But then someone posts about a 27-year-old and that gives me hope.”
Rocio also continues to take her family’s journey one day at time – after all, she’s adjusting a new reality of parenting a medically complex 4-year-old as well as a 2-year-old daughter, who is not a carrier for Sanfilippo.
“I went from having a life to having a totally different life,” she says.