Oliver’s Journey to a Diagnosis
Oliver Marley was born at 33 weeks after a complicated pregnancy for his mother Caroline, whose placenta partially detached from her uterus when she was 14 weeks pregnant.
Born weighing 5 pounds and 4 ounces, Oliver had bruises over much of his body and had to be intubated a day after birth when he went into respiratory failure. Doctors detected a small brain bleed and noticed that, at 6 days old, both of his middle fingers were contracted.
Oliver began treatment at another hospital, where doctors suspected he might have muscular dystrophy. Their outlook for Oliver was grim and they suggested he might need to be sent to an acute-care facility.
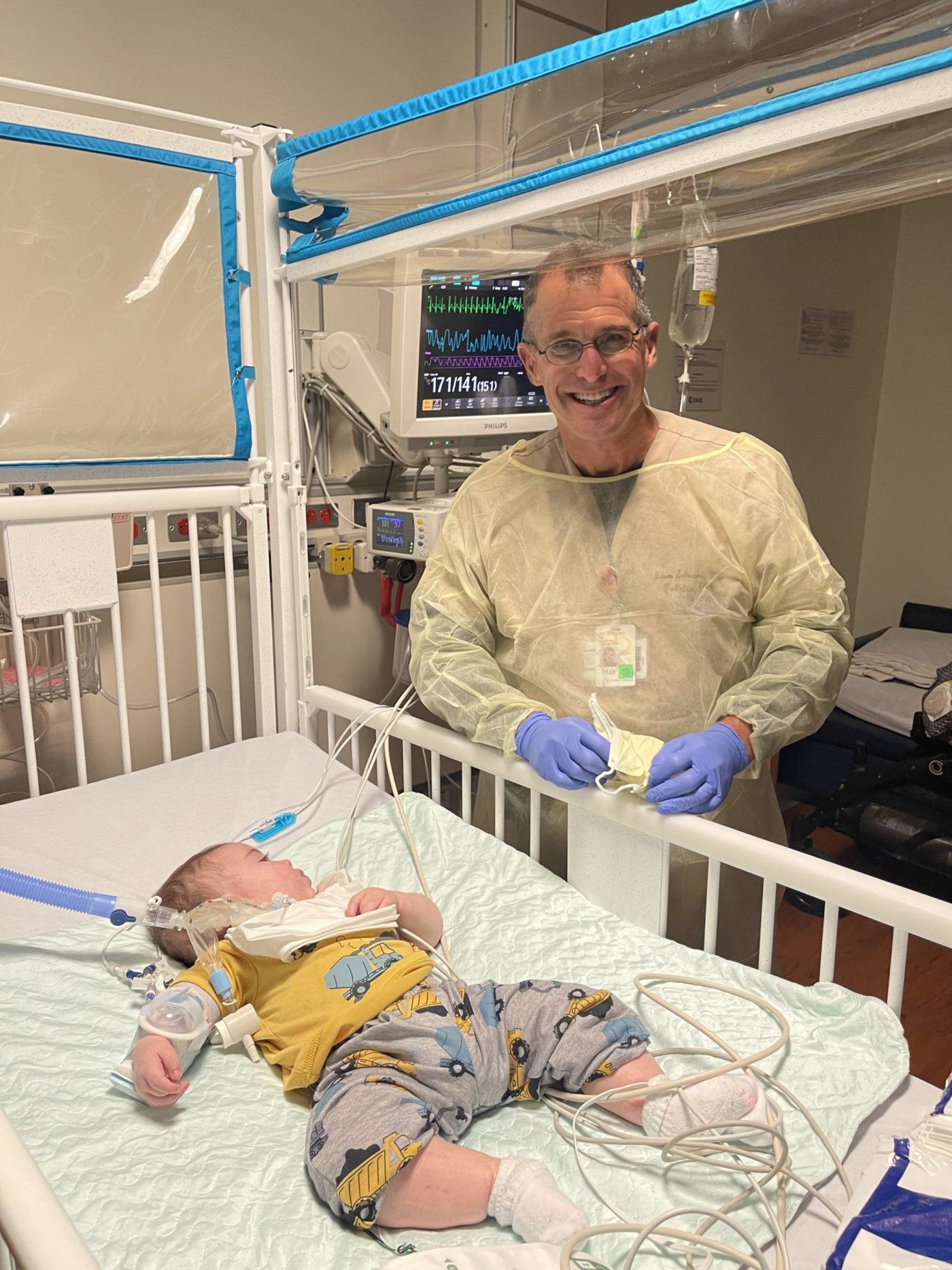
That outlook changed though on Aug. 11, 2020, when Oliver was transferred to CHOC. At 8 weeks old, He underwent a tracheotomy and was attached to a ventilator.
“He literally started thriving,” Caroline recalls. “He started growing because he was not working so hard to breathe. You could just see he was doing better.”
Still without a diagnosis, Oliver went home on Oct. 19, 2020 with a tracheostomy tube and a ventilator.

He returned to CHOC after he contracted a viral infection.
Not convinced Oliver had muscular dystrophy, the CHOC team turned to rapid whole genome sequencing (rWGS) to find out what was really happening in his little body.
Delivering hope, answers
If a Major League Baseball player were to step up to the plate 150 times and get a hit 76 times, his batting average would be an unthinkably torrid .507.
When it comes to identifying genetic causes for some of the rarest and serious diseases in children, CHOC has put up numbers that even Mike Trout couldn’t dream of achieving.
Since July 2017, CHOC has ordered the comprehensive and cutting-edge test of rWGS on 150 patients, with 76 of them getting a precise diagnosis that, in many cases, has resulted in life-changing care.
“We took what could have been a diagnostic odyssey for these patients and families and cut it down from weeks, months, and sometimes years to, in some cases, only three days,” says CHOC pediatric intensive care unit medical director Dr. Jason Knight, part of an informal leadership team that oversees treatment of critically ill kids with rare diseases in the NICU, PICU and CVICU. Other ICU physician team leaders include Dr. Adam Schwarz, Dr. Juliette Hunt and Dr. John Cleary.
CHOC’s rWGS research program was championed by the late Dr. Nick Anas, a CHOC pediatrician-in-chief who was director of pediatric intensive care and a beloved figure at the hospital. Dr. Anas, who started at CHOC in 1984, died on April 3, 2018.
Dr. Anas’ vision for the rWGS research program continues to be realized with successful patient outcomes, from the 2019 diagnosis of an infant girl with the extremely rare cardiac condition Timothy Syndrome to, more recently, Oliver.
“The CHOC team believed in Oliver – they loved him and took care of him and saw worth in him,” says Caroline. “They told me, ‘We want you to take your baby home,’” Caroline says.
Testing began in 2017
Each of us has some 22,000 genes in our bodies that dictate things ranging from the color of our hair to whether we are tall or short. Genes also produce the proteins that run everything in our bodies. Although individually rare, there are more than 6,200 single-gene diseases. rWGS is the technology that, with just a teaspoon of our blood, allows us to look at all the genes in our cells.
At CHOC, rWGS testing became prominent with the launch of Project Baby Bear in fall 2018. CHOC was among five hospitals to participate in that program, led by Rady Children’s Institute for Genomic Medicine (RCIGM) in San Diego. RCIGM has a lab that runs sequencing.
“To have (the RCIGM) close by and to be a close partner with them has been great,” Dr. Knight says. “We are way ahead of many other pediatric hospitals in this area. It’s a great success story, and something I’m really glad to be a part of.”
A total of 45 CHOC patients got tested through Project Baby Bear, a $2-million state program for critically ill infants age 1 or younger who were enrolled in Medi-Cal. Of those 45 patients, 55.6 percent – 25 children – were able to have their rare diseases properly diagnosed, says Dr. Neda Zadeh, a CHOC medical geneticist who was involved with setting up CHOC’s rWGS program with Dr. Anas and who has seen most of the 150 kids tested thus far.
CHOC actually began ordering rWGS testing on patients the year before in a partnership with RCIGM and Illumina, a leading developer and manufacturer of life science tools and integrated systems for large-scale analysis of genetic variation and function. In that 2017 program, 82 CHOC patients were tested with a 47.6 percent positive diagnosis rate, says Ofelia Vargas-Shiraishi, a senior clinical research coordinator in critical care/neonatology research at CHOC.
CHOC has paid for an additional 23 children to undergo rWGS testing outside of the now-completed Ilumina and Project Baby Bear programs, and has funding to pay for up to about six children every year to get tested, says Dr. Schwarz.

“In the long run,” Dr. Schwarz says, “we’re saving money by avoiding expensive workups.”
Adds Dr. Knight: “For a lot of these families, having an answer – even one they might not want to hear – is extremely important.”
For parents like Caroline, the results have been priceless.
A diagnosis for Oliver
Three days later, in mid-November 2020, the Marleys received an answer: Oliver had two extremely rare genetic changes in his AHCY gene that potentially resulted in S-AdenosylHomocysteine Hydrolase (SAHH) deficiency.
It is an extremely rare condition with less than 30 patients reported in the world and CHOC’s Dr. Richard Chang, a metabolic disorders specialist and biochemical geneticist, was consulted to confirm the diagnosis. The disease, which affects brain, muscle and liver development, is associated with high blood levels of methionine and extremely high levels of toxic S-AdenosylHomocysteine (SAH) that interferes with vital cellular growth.
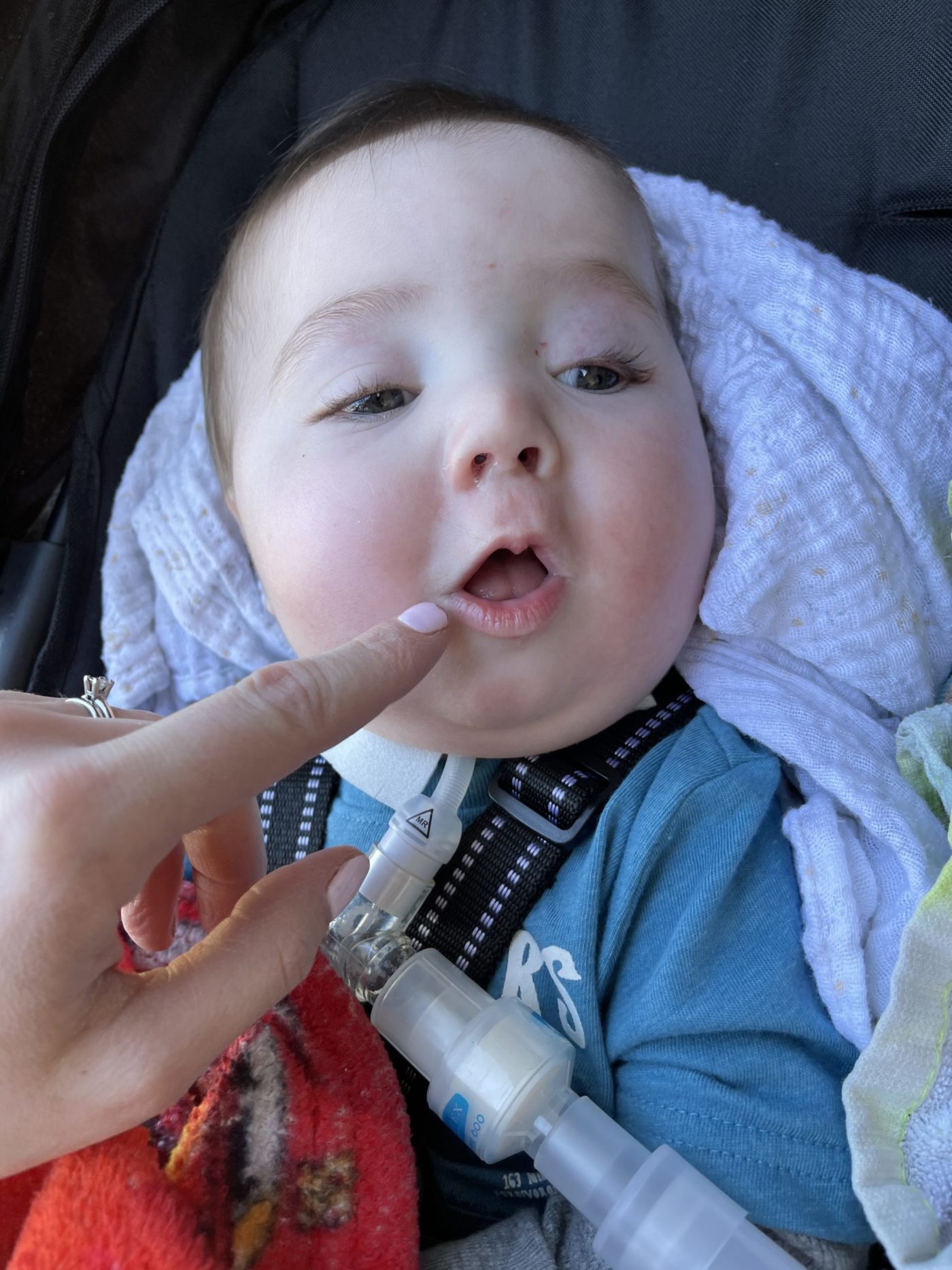
Oliver was put on a delicate protein-restricted diet to limit the production of SAH without causing protein malnutrition, and his condition immediately improved. Other medications were added subsequently to provide nutrients that deficient due to the toxicity of SAH. He has a condition that is identical to a girl in Pennsylvania who was diagnosed at age 3 and later underwent a liver transplant. That girl is now 9.
Oliver is scheduled to receive a liver transplant soon, Caroline says.
Expanding access to rWGS testing
A lawmaker in San Diego, in partnership with Rady Children’s Hospital and Health Center, is pushing for a new law that would expand access to rWGS testing by qualifying it as a Medi-Cal covered benefit for babies hospitalized in intensive care.
Assembly Bill 114, The Rare Disease Sequencing for Critically Ill Infants Act, not only would expand availability of such testing to more families, but also would reduce state spending by eliminating many unneeded procedures, treatments and longer hospital stays, State Assemblyman Brian Maienschein wrote in a recent op-ed piece.
“For critically ill infants hospitalized with unexplained rare diseases,” Maienschein wrote, “the opportunity to benefit from a medical miracle has arrived.”
Caroline sees that miracle daily with Oliver, who now is up to 20 pounds and moving around more.
“We at CHOC are slowly building a case for early introduction of rWGS into the clinical management of these difficult cases in high-acuity settings to improve lifelong clinical outcomes and quality of life,” says Brent Dethlefs, executive director of the CHOC Research Institute.
“There’s growing evidence that early introduction of this technology results in overall cost savings,” Brent adds. “It’s important to get more insurance carriers to cover the cost of this testing over time, which will make rapid whole genome sequencing more available to vulnerable and underserved populations. CHOC always has been an advocate for social justice in health care, which includes greater access to genomic testing.”
Caroline praises the entire collaborative team at CHOC and the entire CHOC Specialists Metabolic Disorders division, including Dr. Chang, who is in charge of maintaining Oliver’s health until transplant; Erum Naeem, clinical research coordinator, NICU; and Cathy Flores, clinical research nurse coordinator, critical care.
“It was a team effort involving the critical care, neonatology, metabolic and genetics teams, just to name a few, and a very strong partnership with RCIGM,” says Ofelia Vargas-Shiraishi, a clinical research coordinator at CHOC.
“We had everyone by our side every step of the way,” Caroline adds. “Child life was amazing, and so is the spiritual care team. If you’re willing to learn, they’re willing to teach you.”
Dr. Zadeh says the success of CHOC’s rWGS program – with its whopping .507 batting average – is a result of “a very unique blend of the right people coming together at the right time and the right institution with the right set-up.”
She adds, “I don’t think it would have worked necessarily at every hospital. I think CHOC is unique. We have the right group of kids we are testing. And we have the right group of specialists involved.
“We love our families. We get to have really great relationships with them. This program just shows that CHOC is all about the whole care of the child and the family.”